 |
Pathogen-induced Expression of IFNs and ISGs |
Innate immune recognition is mediated by a system of germline-encoded receptors (Toll receptors, TLRs) that recognize conserved molecular patterns associated with microbial pathogens. These receptors, which are coupled to downstream signaling cascades that mediate the induction of immune-response genes, represent the most ancient host defense system found in mammals, insects and plants. |
 |
Micro-RNAs in the Mammalian Host Defense |
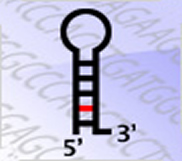
RNA interference through non-coding microRNAs (miRs) represents a vital component of the innate antiviral immune response in plants and invertebrate animals, however, a role for cellular miRs in the defense against viral infection in mammalian organisms has thus far remained elusive. We found that interferons or TLR activation rapidly modulates the expression of numerous cellular miRs. Several of these IFN-induced miRs have predicted targets within viruses such as hepatitis C virus (HCV), HIV, Influenza or Dengue virus. We recently demonstrated the antiviral activity of several cellular miRs against HCV infection. There, introduction of synthetic miR-mimics corresponding to these IFN-induced miRs reproduces the antiviral effects of IFN on HCV replication and infection, whereas neutralization of these antiviral miRs reduces the antiviral efficacy of IFN. We are currently investigating the antiviral capacity of the IFN-induced miRs against several other viruses. Taken together, our findings strongly support the notion that mammalian organisms too, via the interferon system, utilize cellular miRs to combat viral infections. |
 |
Interferons and Autoimmune Disorders |
|
